Spinning Disk W1
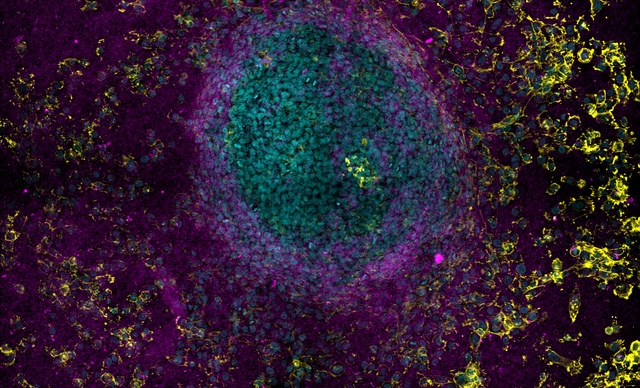
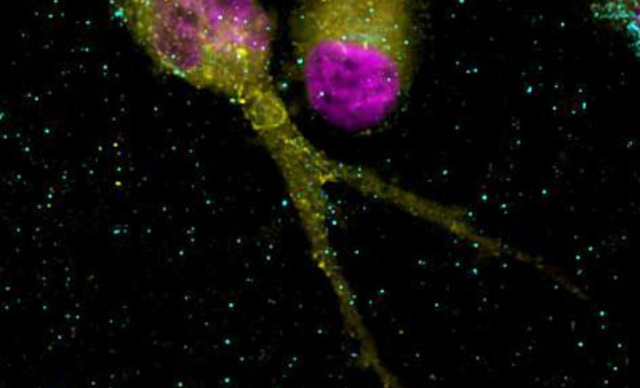
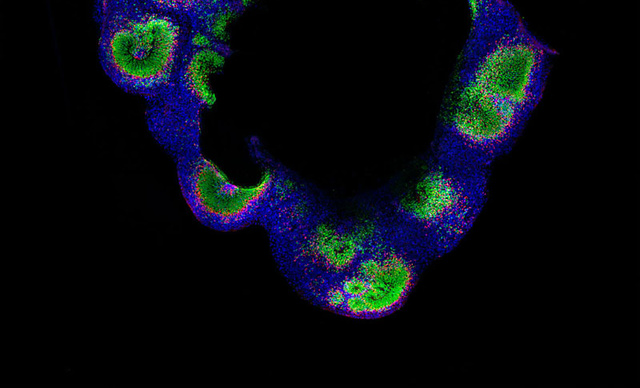
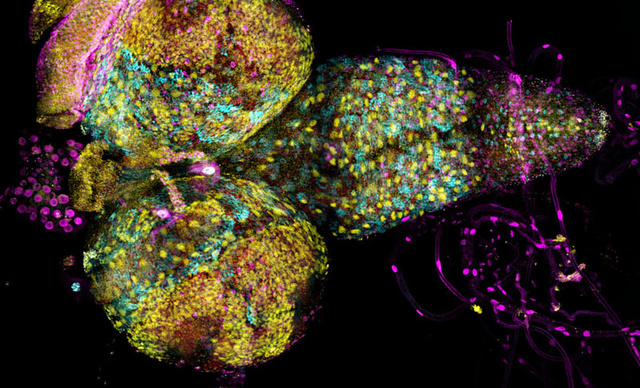
The scanning confocal spinning disks systems integrated with Nikon’s microscope platforms and optics, allow users flexible and powerful systems for a wide range of imaging applications.
Field scanners are renowned for their low dosage, specimen-friendly characteristics, making them ideal for live cell or organism applications.
Field scanners are renowned for their low dosage, specimen-friendly characteristics, making them ideal for live cell or organism applications.